Solid oxide fuel cells (SOFC) are electrochemical energy converter which operate at temperatures as high as 800°C - 1000°C. SOFC are based predominantly on ceramic components and can combust - unlike the standard PEM fuel cells - affordable hydrocarbon fuels and convert them into electricity plus heat. Yet, a number of issues keeps SOFC from becoming a normal consumer product.
You can read up my work on SOFC in the papers shown below. A more comprehensive view is available in my book chapter on SOFC characterization techniques with Lorenzo Malavasi.
A complete overview on SOFC can be found in my book with de Gruyter.
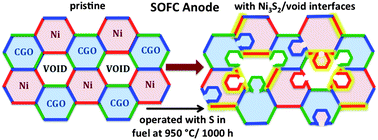
Sulfur poisoning and microstructure degradation in SOFC anodes
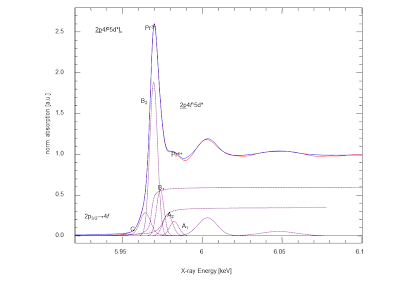
Impurities in fuels such as sulfur interact adversely with the SOFC anodes. There is not very much know about the sulfur chemistry with respect to SOFC operation. We have applied x-ray absorption spectroscopy at the sulfur K-edge in order to learn about the sulfur moeities on SOFC anodes after operation. The anodes we looked at had a strong sulfate signature, but also signature from sulfo-organic species such as thiophene, which is kind of unexpected given that SOFC operate at temperatures around 800°C. Below on the left you see the first x-ray absorption sulfur near-edge structure (XANES) spectrum of an operated SOFC anode from Forschungszentrum Jülich. In the middle you see an exemplary SOFC cell assembly of 200 micron thickness [3]. During operation, the microstructure of the SOFC components undergo chemical changes which trigger microstucture changes which adversely impact the SOFC stability. We use ultra small angle x-ray scattering (USAXS) for the quantitative analysis of microstructure changes [4].
Figure 1: left - Sulfur XANES of SOFC anode, highlighting sulfur moeities. middle - SOFC cell assembly with 200 micrometer thickness, showing anode, electrolye, and cathode. right - Size distribution of objects in SOFC anode obtained with ultra small angle x-ray scattering.
In the last few years we (with Rudolf Struis from PSI and Gunnar Nurk from Tartu University) succeeded to make the ever first operando sulfur XANES on a running SOFC at the Swiss Light Source in Villigen PSI [2]. We (Jan Ilavsky from APS, Andrew Allen from NIST and Pete R. Jemian, APS) have also improved our USAXS experiments to the extent that we use anomalous small angle x-ray scattering (ASAXS). This method allows for enhancement of chemical contrast in the SOFC electrode assemblies and thus directly access the important triple phase boundaries [1]. We have tuned the x-ray energies to the Ni K-edge and the Zr K-edge. For this it was necessary to thin the SOFC electrode assemblies down to less than 30 micrometers.
[1] A. J. Allen, J. Ilavsky, P. R. Jemian, A. Braun, Evolution of electrochemical interfaces in solid oxide fuel cells (SOFC): a Ni and Zr resonant anomalous ultra-small-angle X-ray scattering study with elemental and spatial resolution across the cell assembly, RSC Adv., 2014, 4, 4676-4690. http://pubs.rsc.org/en/content/articlelanding/2013/ra/c3ra46886k#!divAbstract
[2] G. Nurk, T. Huthwelker, A. Braun, Chr. Ludwig, E. Lust, R. P. W. J. Struis, Redox dynamics of sulphur with Ni/GDC anode during SOFC operation at mid- and low-range temperatures: An operando S K-edge XANES study, J. Power Sources 2013, 240, 448–457 http://www.sciencedirect.com/science/journal/aip/03787753
[3] A Braun, M. Janousch, J. Sfeir, J. Kiviaho, M. Noponen, F. E. Huggins, M. J. Smith, R. Steinberger-Wilckens, P. Holtappels, T. Graule, Molecular speciation of sulfur in solid oxide fuel cell anodes with x-ray absorption spectroscopy, J. Power Sources 2008,183, 2, 564-570. http://www.sciencedirect.com/science/article/pii/S0378775308009671
[4] A.J. Allen, J. Ilavsky, A. Braun, Multi-Scale Microstructure Characterization of Solid Oxide Fuel Cell Assemblies with Ultra Small-Angle X-Ray Scattering, Advanced Engineering Materials 2009, 11 (6), 495-501. http://www3.interscience.wiley.com/journal/122443526/abstract
Ceramic proton conductors for intermediate temperature SOFC
The electrolytes in SOFC are typically oxygen ion conductors which need a temperature as high as 800°C for operation. Proton conductors operate at significantly lower temperaturs such as 400°C - 600°C.
[3] A Braun, S. Duval, J.P. Embs, F. Juranyi, P. Ried, P. Holtappels, R. Hempelmann, U. Stimming, Th. Graule. Proton diffusivity in the BaZr0.9Y0.1O3-delta proton conductor. J. Appl. Electrochem. 2009, 39(4), 471-475.
[4] A Braun, A. Ovalle, S. Erat, V. Pomjakushin, A. Cervellino, W. Stolte, and T. Graule, Yttrium and hydrogen superstructure and correlation of lattice expansion and proton conductivity in the BaZr0.9Y0.1O2.95 proton conductor, Appl. Phys. Lett., 95, 224103, 2009.
[5] S. Erat, A. Braun, A. Ovalle, C. Piamonteze, Z. Liu, T. Graule, L.J. Gauckler, Correlation of O(1s) and Fe(2p) NEXAFS spectra and electrical conductivity of La1-xSrxFe0.75Ni0.25O3-δ, Appl. Phys. Lett., 95(17), 174108, 2009.
[6] Q. Chen, A. Braun, A. Ovalle, C.-D. Savaniu, T. Graule, N. Bagdassarov, Hydrostatic pressure decreases the proton mobility in the hydrated BaZr0.9Y0.1O3 proton conductor, Appl. Phys. Lett. 97, 041902 (2010)
[7] Q. Chen, A. Braun, S. Yoon, N. Bagdassarov, T. Graule, Effect of lattice volume and compressive strain on the conductivity of BaCeY-oxide ceramic proton conductors, J. Eur. Ceram. Soc. (2011) 31 (14) 2657-2661.
Spectroscopy & scattering on LaSrFeNi-oxide for intermediate temperature SOFC
This is mainly for the correaltion of conductivity and spectral characteristics as a function of substitution parameter and also as temperature.
[8] A. Braun, S. Erat, A. Ariffin, R. Manzke, H. Wadati, T. Graule, L.J. Gauckler, High temperature oxygen NEXAFS valence band spectra and conductivity of LaFe3/4Ni1/4O3 from 300 K to 773 K, Appl. Phys. Lett. 2011, 99 (21) pp.
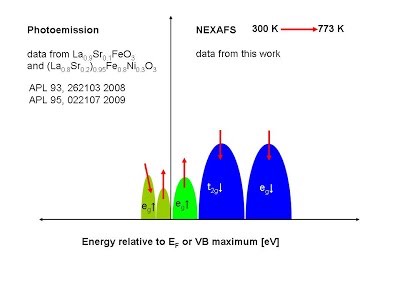
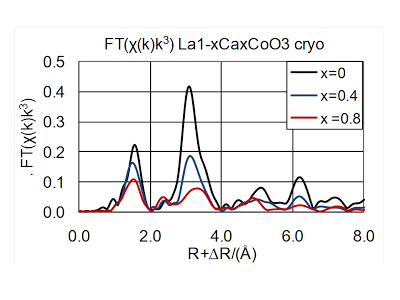
[9] O. Haas, C. Ludwig, U. Bergmann, R.N. Singh, A. Braun, T. Graule, X-ray absorption investigation of the valence state and electronic structure of La1-xCaxCoO3-δ in comparison with La1-xSrxCoO3-δ and La1-xSrxFeO3-δ; J. Solid State Chem. http://dx.doi.org/10.1016/j.jssc.2011.09.027
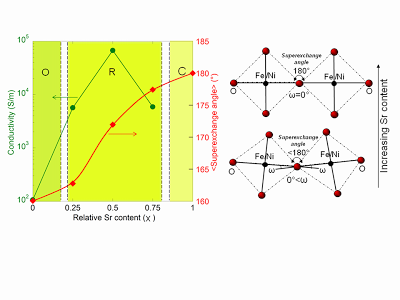
[10] S. Erat, A. Braun, C. Piamonteze, Z. Liu, A. Ovalle, H. Schindler, T. Graule, L. J. Gauckler. Entanglement of charge transfer, hole doping, exchange inter-action and octahedron tilting angle and their influence on the conductivity of La1-xSrxFe0.75Ni0.25O3: A combination of x-ray spectroscopy and diffraction, J. Appl. Phys. 108, 124906 (2010) http://jap.aip.org/resource/1/japiau/v108/i12/p124906_s1 http://arxiv.org/abs/1106.1029
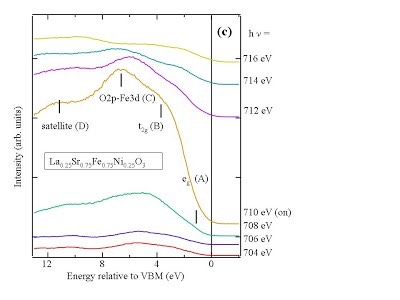
[11] S. Erat, H. Wadati, F. Aksoy, Z. Liu, T. Graule, L.J. Gauckler, A. Braun, Fe-resonant valence band photoemission and oxygen NEXAFS study on La1-xSrxFe0.75Ni0.25O3-δ, Appl. Phys. Lett. 97, 124101 (2010); http://dx.doi.org/10.1063/1.3484960 http://arxiv.org/abs/1106.1086
[12] A. Braun, S. Erat, R. Mäder, X. Zhang, Y. Sun, Z. Liu, Bongjin S. Mun, M. Ari, H. Grimmer, E. Pomjakushina, S.S. Mao, K. Conder, L.J. Gauckler, T. Graule, Quantitative correlation of bulk conductivity and surface representative valence band characteristics in iron perovskites for 300 K < T < 800 K: High temperature photoemission studies on single crystal monoliths and films; J. Electron Spectroscopy and Related Phenomena 181 (2010), pp. 56-62; http://dx.doi.org/10.1016/j.elspec.2010.05.024 http://arxiv.org/abs/1106.1034
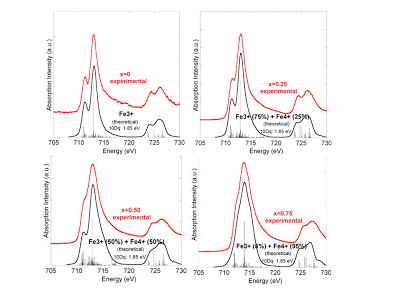
[13] S. Erat, A. Braun, A. Ovalle, C. Piamonteze, Z. Liu, T. Graule, L.J. Gauckler, Correlation of O(1s) and Fe(2p) NEXAFS spectra and electrical conductivity of La1-xSrxFe0.75Ni0.25O3-δ, Appl. Phys. Lett., 95(17), 174108, 2009. http://apl-beta.aip.org/applab/v95/i17/p174108_s1 http://arxiv.org/abs/1106.1019
[14] A Braun, X. Zhang, Y. Sun, U. Müller, Z. Liu, S. Erat, M. Ari, H. Grimmer, S.S. Mao, T. Graule, Correlation of high temperature X-ray photoemission valence band spectra and conductivity in strained LaSrFeNi-oxide on SrTiO3(110), Applied Physics Letters, 95, 022107, 2009.
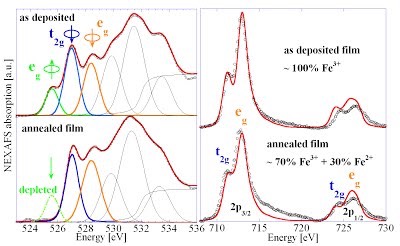
[15] A Braun, D. Bayraktar, A.S. Harvey, D. Beckel, J.A. Purton, P. Holtappels, L.J. Gauckler, T. Graule, Pre-edges in oxygen (1s) x-ray absorption spectra: A spectral indicator for electron hole depletion and transport blocking in iron perovskites, Applied Physics Letters 94 (20), 202102, 2009. http://link.aip.org/link/?APPLAB/94/202102/1 http://arxiv.org/abs/1106.0991
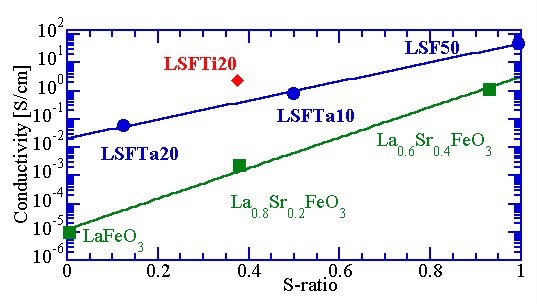
[16] A. Braun, J. Richter, A. S. Harvey, S. Erat, A. Infortuna, A. Frei, E. Pomjakushina, Bongjin S. Mun, P. Holtappels, U. Vogt, K. Conder, L. J. Gauckler, and T. Graule. Electron hole–phonon interaction, correlation of structure, and conductivity in single crystal La0.9Sr0.1FeO3, Applied Physics Letters 93, 262103, 2008. http://link.aip.org/link/?APPLAB/93/262103/1 http://arxiv.org/abs/1106.1014
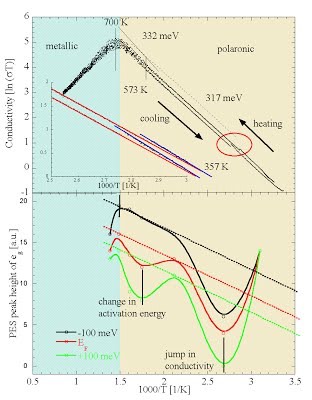
[17] O. Haas, U.F. Vogt, C. Soltmann, A. Braun, W.-S. Yoon, X.Q. Yang, T. Graule. The Fe K-edge X-Ray Absorption Characteristics of La1-xSrxFeO3-δ Prepared by Solid State Reaction. Materials Research Bulletin 44 (2009), pp. 1397-1404.http://www.science-direct.com/science/article/B6TXC-4V47CNT-1/2/b5da0f2079b8eb21a15eb94d5a39712f
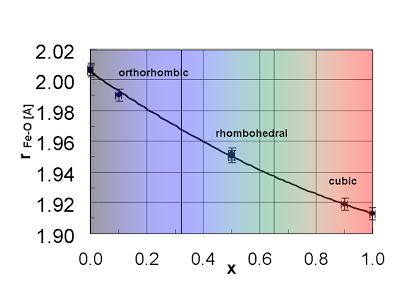
[18] P. Holtappels, A. Braun, T. Thünemann, U. Vogt. Characterization of mixed conducting La-Sr-Fe oxides for application in solid oxide fuel cells. ECS Transactions - Solid Oxide Fuel Cells" Volume 7 (1) 1025-1031 (2007).
[19] J. Richter, A. Braun, A.S. Harvey, P. Holtappels, T. Graule, L.J. Gauckler, Valence changes of manganese and praseodymium in Pr(1–x)Sr(x)Mn(1–y)In(y)O(3–δ) perovskites upon cation substitution as determined with XANES and ELNES. Physica B 2008, 403(1) 87-94. http://www.science-direct.com/science/article/B6TVH-4PCXGHP-3/2/ef0808643988b85125b872b346defdd1